EpiSwitch® Prostate Cancer Detection (PSE) Test
Get the Test
Testing with a standard PSA test is 55% accurate
PSE boosts prostate cancer detection to 94% accuracy
Learn more about
EpiSwitch® Prostate Cancer Detection (PSE) Test
Watch PSE Video1 in 8 men will get prostate cancer in their lifetime
If you have received an elevated PSA result, or have concerns where prostate cancer must be ruled out, talk to your primary care doctor about the PSE test
Fast, accurate results from a routine blood test
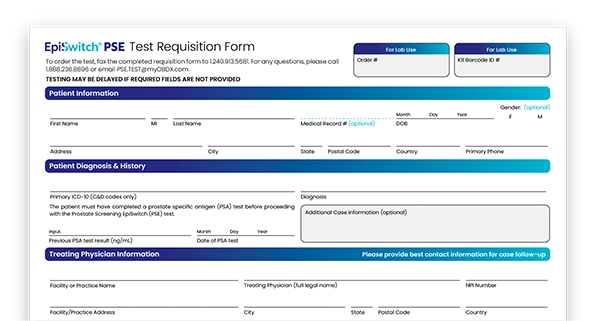
- Complete a Requisition Form – Work with your physician to complete and fax the PSE requisition form to: +1 240-913-5681
- Provide a small blood sample – Your physician will collect and send us a small blood sample using our Specimen Submission Kit
- Receive test results – In approximately 5 days after receipt of the patient’s blood sample, the PSE test result will be electronically & securely reported to the ordering physician.
PSE can reduce referrals for unnecessary prostate biopsies
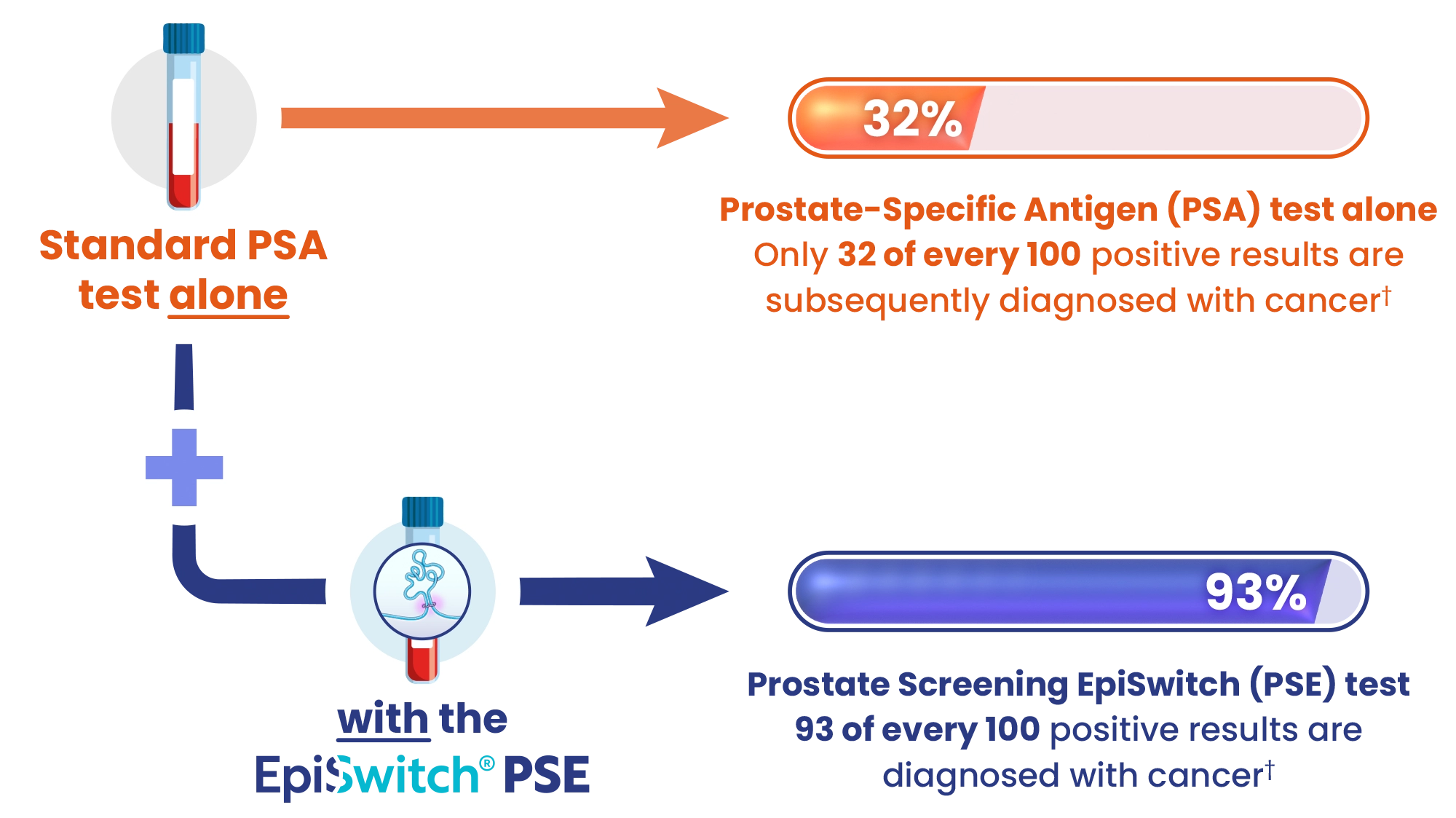
Source: † Pchejetski et al. (2023). Circulating CCS Significantly Enhance PSA PPV and Overall Accuracy for Prostate Cancer Detection. Cancers, 15(3), 821. http://dx.doi.org/10.3390/cancers15030821
Personalized EpiSwitch PSE Report
Personalize your guidance for each patient
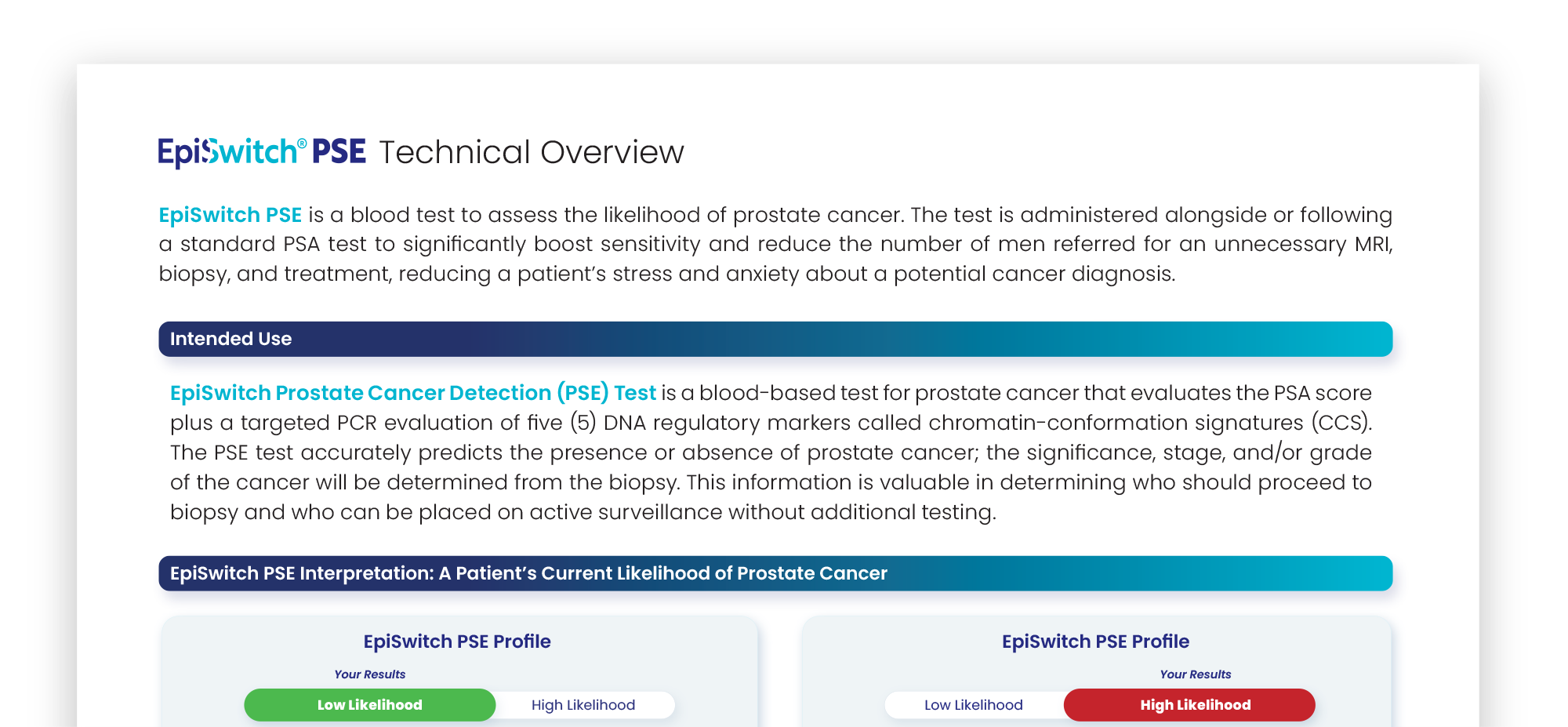
An EpiSwitch PSE report is securely returned to the ordering physician. It includes indications for the healthcare professional to determine who should proceed to biopsy, and who can be placed on active surveillance.
View Sample ReportEpiSwitch PSE Profile
The EpiSwitch Prostate Cancer Detection (PSE) Test result indicates a high likelihood of prostate cancer. This result is consistent with cancer being confirmed in more than 9 out of 10 men.
Your healthcare provider will consider this result along with other clinical features and history to determine which additional diagnostic test(s), such as a biopsy, is appropriate to confirm.
Validated test with high accuracy, specificity and sensitivity
- EpiSwitch PSE is administered alongside or following a standard PSA test
- Blood test measures five (5) epigenetic biomarkers and combines these with the patient’s PSA score
- EpiSwitch PSE was validated across a population of asymptomatic men randomly invited for cancer cancer detection
Download the Technical Overview to learn more about how EpiSwitch PSE can help guide treatment decisions for patients and their providers.
Download Technical OverviewFast and accurate prediction of an individual's likelihood of prostate cancer with a routine qPCR blood test.
Our comprehensive and validated approach delivers a report to the ordering physician via secure electronic communication or fax. Results will be available around 5 days after the lab receives the patient's blood sample.
Built on Oxford BioDynamics' EpiSwitch technology platform, PSE is based on a comprehensive and validated approach
The EpiSwitch PSE blood signature was developed using Oxford BioDynamics' EpiSwitch 3D genomic immune health database—the world's largest genomic database—via several controlled studies.
PSE uses 3D genomic profiling to assess an individual's current likelihood of prostate cancer. This method uncovers vital drivers of systemic changes in their blood, including immune cells associated with prostate cancer.
Get The TestAre you interested in becoming an EpiSwitch PSE registered healthcare provider?
Contact EpiSwitch PSE Customer Service at 888-236-8896 (US) or 01865 504932 (UK) to open an account, request customized EpiSwitch PSE Requisition Forms branded for your practice, order EpiSwitch PSE collection kits, or request further assistance relating to EpiSwitch PSE. We look forward to speaking with you.
Download the Requisition Form and order your patient's test today.
Download the Requisition Form (US) Download the Requisition Form (UK)